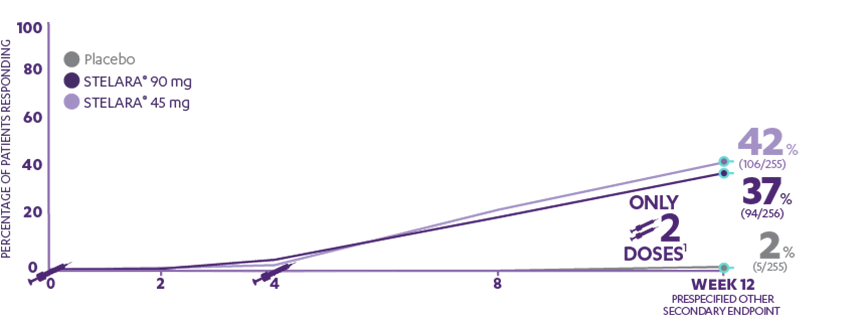
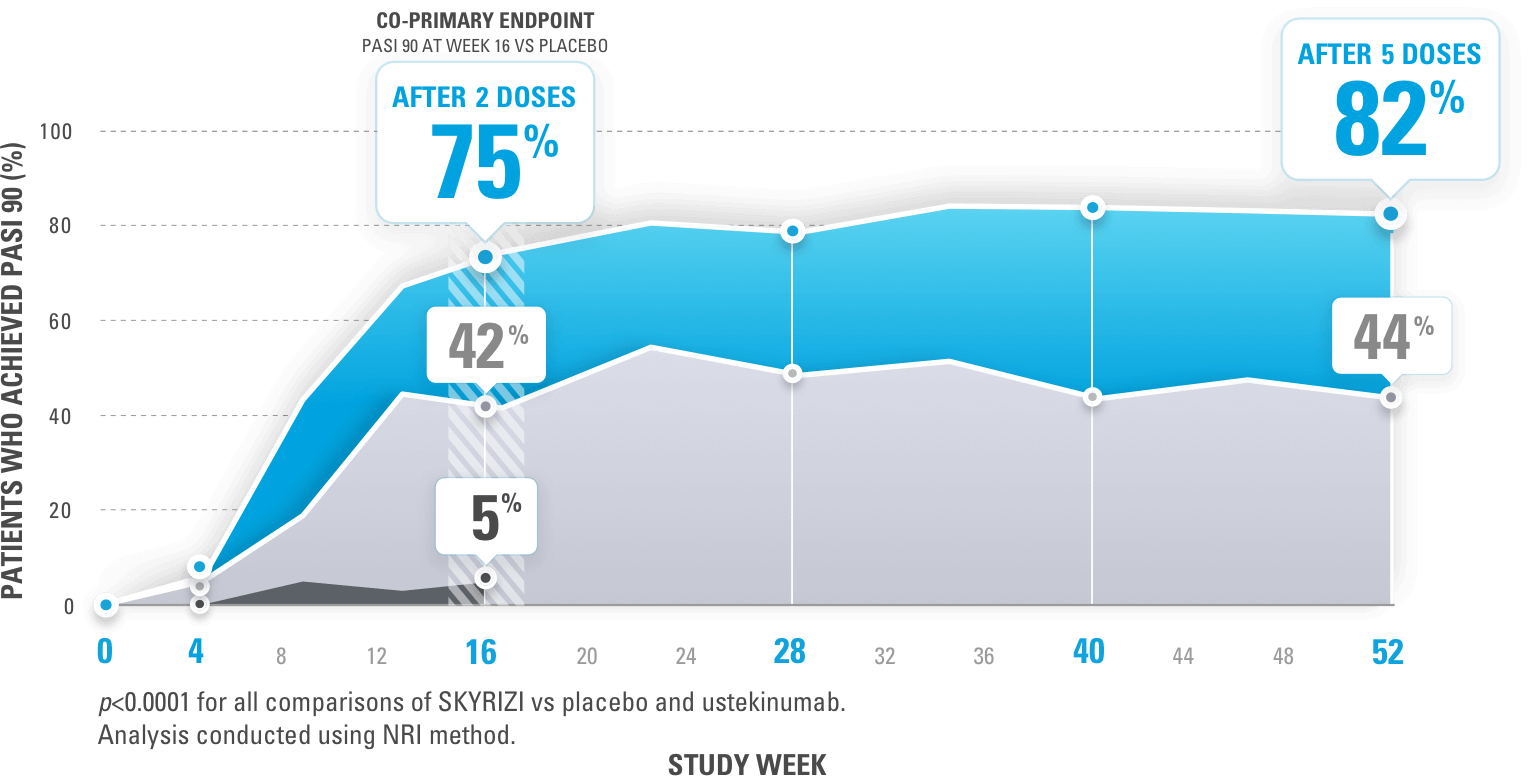
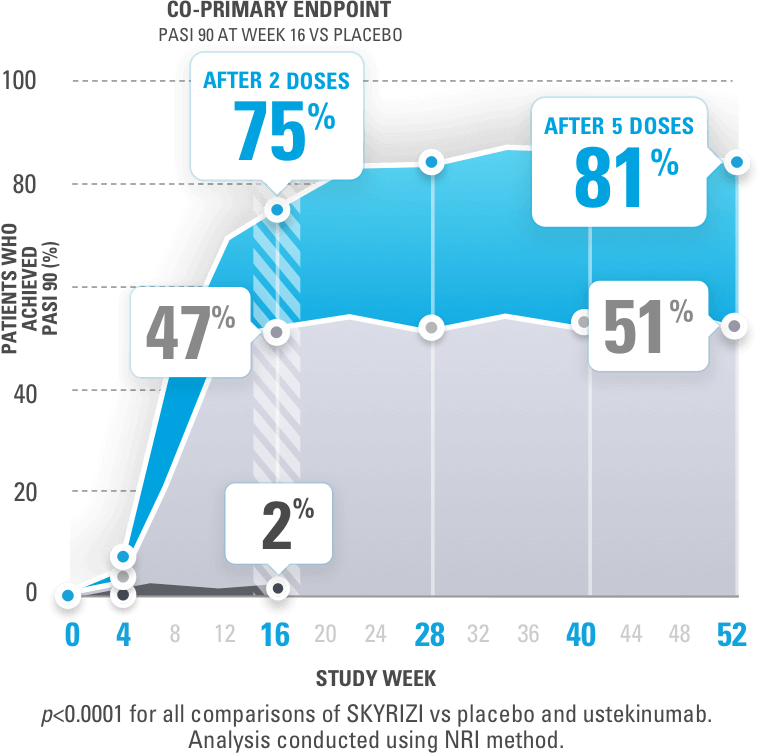
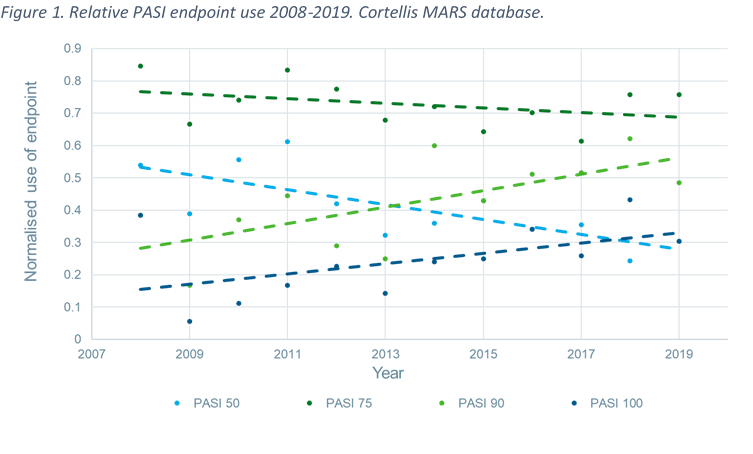
A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis - ScienceDirect

Time to Raise the Bar to Psoriasis Area Severity Index 90 and 100 - JDDonline - Journal of Drugs in Dermatology

Tildrakizumab in Patients with Moderate-to-Severe Psoriasis: Post-Hoc Analyses on Efficacy, Time to Relapse, Long-Term Safety in Elderly Population and Predictability From The reSURFACE 1 and reSURFACE 2 Phase III Clinical Trials -

PASI 75, PASI 90, and PASI 100 response by baseline psoriasis severity... | Download Scientific Diagram

Figure 1 | Rapid Response of Biologic Treatments of Moderate-to-Severe Plaque Psoriasis: A Comprehensive Investigation Using Bayesian and Frequentist Network Meta-analyses | SpringerLink

Secukinumab demonstrates high efficacy and a favorable safety profile over 52 weeks in Chinese patients with moderate to severe plaque psoriasis | Chinese Medical Journal
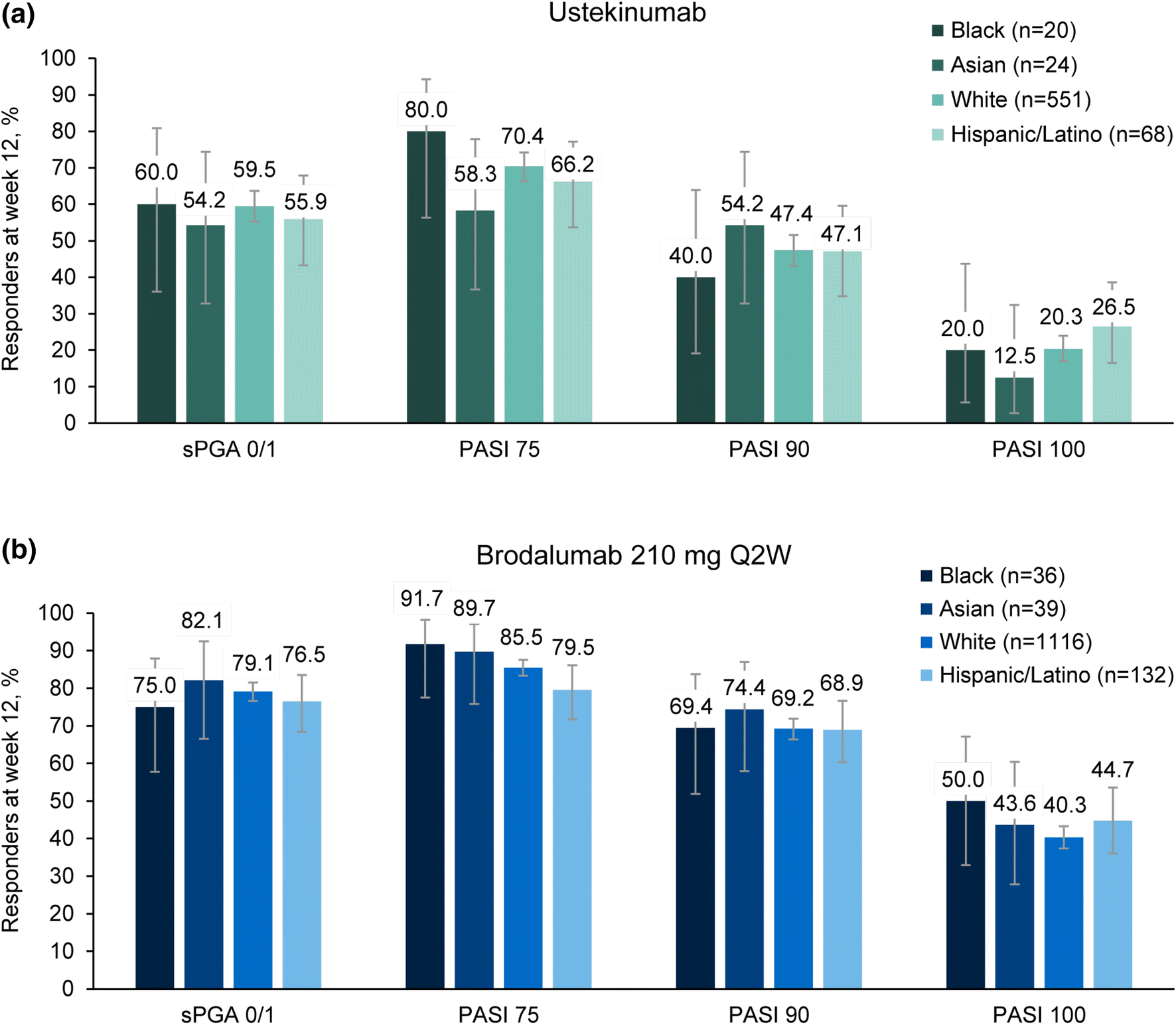
Figure 2 | Efficacy and Safety of Brodalumab in Patients with Moderate-to-Severe Plaque Psoriasis and Skin of Color: Results from the Pooled AMAGINE-2/-3 Randomized Trials | SpringerLink
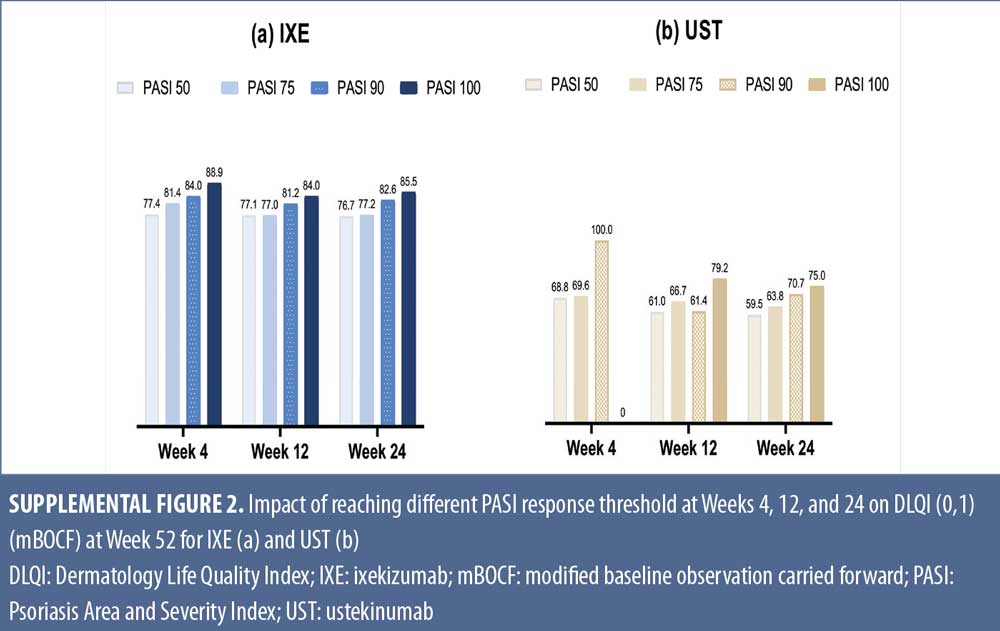
Early Treatment Targets for Predicting Long-term Dermatology Life Quality Index Response in Patients with Moderate-to-Severe Plaque Psoriasis: A Post-hoc Analysis from a Long-term Clinical Study – JCAD | The Journal of Clinical

PASI 90 / 100 , DLQI 0 / 1 , and IL-17 Receptor / Cytokine : Does it Make a Difference and Are We Ambitious Enough ? | Semantic Scholar

Secukinumab 2‐weekly vs. 4‐weekly dosing in patients with plaque‐type psoriasis: results from the randomized GAIN study* - Reich - 2021 - British Journal of Dermatology - Wiley Online Library

Secukinumab dosing every 2 weeks demonstrated superior efficacy compared with dosing every 4 weeks in patients with psoriasis weighing 90 kg or more: results of a randomized controlled trial* - Augustin -